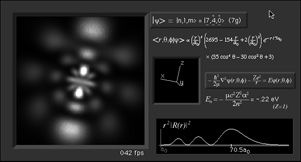
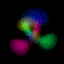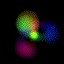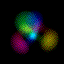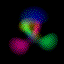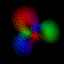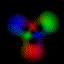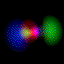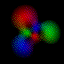Real-Time Visualization of the Quantum Mechanical Atomic Orbitals The orbital images on this page represent the shape of the atomic orbitals. The clouds you see are the probability distribution of an electron bound to a Hydrogen nucleus. These images were created using Atom in a Box (PowerPC Macintosh only, v1.0.8), a scientific and educational program that aids in visualizing the Hydrogenic atomic orbitals, a prime and otherwise unwieldy example of quantum mechanics.
Unlike other tools in this category, this program raytraces through a three-dimensional cloud density that represents the wavefunction's probability density and presents its results in real-time (up to 48 frames per second on the latest hardware). The user interface is very interactive and provides a wide degree of flexibility. It contains all 140 eigenstates up to the n=7 energy level and the allowed spectral transitions between those eigenstates. It also allows a state formed by a superposition (see below) of up to eight of those eigenstates allowing for over 3 trillion possible states. The program can display a wavefunction as a picture of a cloud, use color as phase, plot in red-cyan left/right for 3D glasses, and slice the wavefunction. You can see more example images at the bottom of the page. What is Quantum Mechanics? One of the great advances in human knowledge of the twentieth century is the birth of the theory of Quantum mechanics. It has led to some of the most common technologies used today, including the little transistors that make up the computers you're using to read this. One of the mysteries it revealed was the structure of the atom. Classical mechanics could not properly explain the existence of the atom. Because there was nothing to stop electrons from spiralling in to the nucleus, it predicted that all atoms would immediately destroy themselves in a spectacular high-energy blast of radiation. Well, that obviously doesn't happen. Quantum mechanics describes that the electron (and all of the universe for that matter) exists in any of a multitude of states. The particular physical situation determines what and how many states there are. Borrowing from some of the techniques in mathematics, physicists organize these states into a particular set of mathematically convenient states called "eigenstates". Eigenstates are good to use because what makes one eigenstate different from another usually has a physical meaning. They also can make an horribly difficult problem managable. These and other phenomena in Quantum mechanics predict that possiblities in physical phenomena have distinct separations (e.g., "quantum leaps") and that energy transport exists as indivisible packets, called "quanta". Hence the name: Quantum Mechanics. What are Orbitals? By applying these techniques to the hydrogen atom, physicists are able to precisely predict all of its properties. The electron eigenstates around the nucleus are called "orbitals", in a rough correspondence with how the Moon orbits the Earth. We find that these states do not allow the electron to crash into the nucleus, but instead find themselves in any combination of these orbital eigenstates. These orbitals' physical structure describe effects from how atoms bond to form compounds, magnetism, the size of atoms, the structure of crystals, to the structure of matter that we see around us. Visualizing these states has been a challenge, because the mathematics that describe the eigenfunctions are not simple and the states are a three-dimensional structures. The standard convention has the orbital eigenstates indexed by three interrelated integer indicies, called n, l, and m. Their range and interdependence comes out of the math in deriving the eigenstates. n can range from 1 to infinity. l can range from 0 to n-1. m can range from -l to +l. They also have physical meaning. The energy of the state, which is negative because the electron is bound to the nucleus, depends only on n and increases as n increases. l refers to the amount of angular momentum the electron has due to its "orbit" around the nucleus. l is not equal to the amount of angular momentum but goes up as angular momentum goes up. m determines how much of the angular momentum is in the z direction. (However, the rest of the angular momentum is not l minus m or anything that simple. That's a long story that I can't fit here. Look in a Quantum textbook (a good one is A Modern Approach to Quantum Mechanics by John S. Townsend), take a course, or talk to a physics professor.)
What's here for me? I have written a Macintosh application that displays atomic orbitals in real-time. Rather than just a plot of the spherical harmonics, as is shown in many Quantum mechanics textbooks, this program displays the electron orbital as a cloud. The cloud's density is determined by the orbital's probability density for the electron. There are three examples on this web page: The one at the top is the n=3, l=2, m=0 state; the screenshot above has n=7, l=4, m=0; the first example below is n=6, l=4, m=1. (The quality is poor so that they wouldn't take too long to download over the Internet. They'll look even worse if you are not using Millions of colors to view it. The program looks quite a bit better, trust me.) With the program, you can rotate the orbital around in real time. If you have red-cyan 3-D glasses, you can see it in 3-D too. The program has all the orbital eigenstates up to n=7, which is the highest occupied shell for the ground states of the heaviest elements, e.g., Uranium, Plutonium, etc. You can download the application, the Atom in a Box, a.k.a. Orbitals, v1.0.8 (~472k PowerPC only, CarbonLib required), from here and have a look. The program is computation intensive, so it requires a PowerPC Macintosh or Macintosh-compatible to run. For individual users, this is US $20 shareware. You can see the details in the about box or the Read Me. Also supplied are example orbital files. v1.0.8 will run on both OS 9 (with CarbonLib) and OS X. The latest pre-Carbon version of Atom in a Box, v1.0.4 is also available. See the READ ME for more details. Atom in a Box, a.k.a. Orbitals, v1.0.4, for 68k is available, but it's really slow (not real-time: 3 seconds per frame on a //ci). A Short Gallery of Animated Orbitals
I hope you like it. ;)
This is http://www.dauger.com/orbitals/ coming to you with no commercial interruptions. |
Back to home. |
This page has been accessed
times. Thanks for stopping by!
|
Copyright © 1997-2008 Dean Dauger - DeanDauger@dauger.com |
|
||